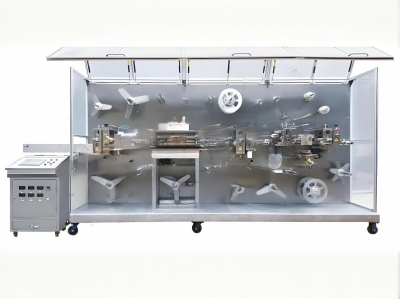
ully Automatic IV Securement Dressing Packaging Machine
Product Overview
Fully Automatic IV Securement Dressing Packaging Machine
Precision Engineered for High-Volume Medical Device Manufacturing
Zowinda's IV Securement Dressing Packaging Machine represents the next generation of automated production solutions for critical medical applications. Designed specifically for catheter fixation devices and advanced wound care products, this system combines:
✔ Unmatched Precision: ±0.1mm accuracy for perfect PU film window cutting
✔ Dual-Material Capability: Seamlessly processes both transparent PU films and nonwoven substrates
✔ Intelligent Automation: IoT-ready architecture with predictive maintenance
✔ Regulatory Ready: Designed for compliance with FDA 21 CFR Part 820 and EU MDR
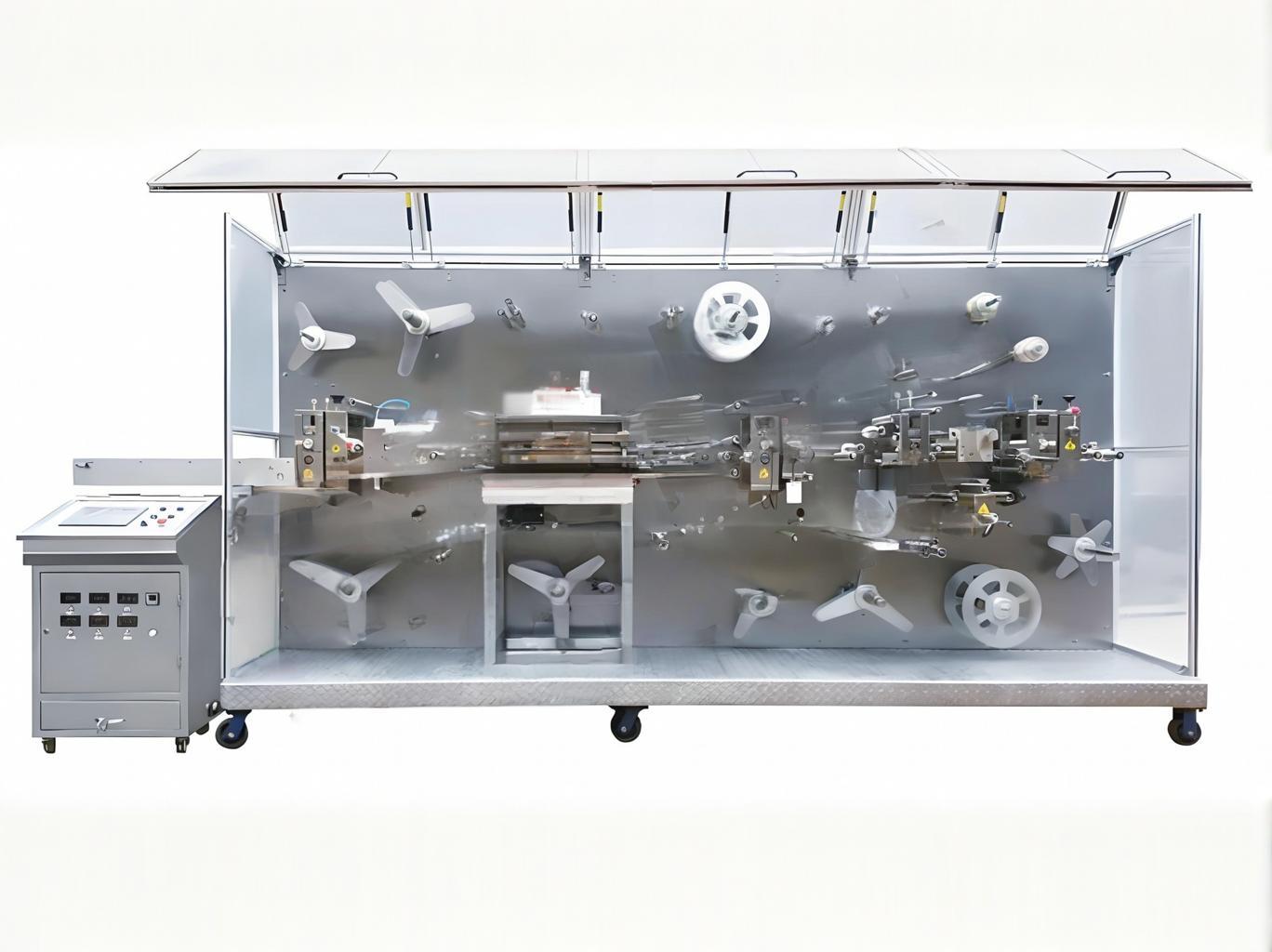
Why Global Medical Manufacturers Choose This Solution
✔ Dual-Material Flexibility - Process both transparent PU films (for IV dressings) and nonwoven fabrics (for general medical dressings)
✔ Pharmaceutical-Grade Sealing - Switch between mold-type (for complex shapes) or roller-type (for high-speed) sealing
✔ Military-Grade Precision - ±0.1mm positioning accuracy with advanced motion control
Core Technology Breakdown
1. Intelligent Motion Control System
Next-generation controller outperforms traditional PLCs with:
→ 0.005mm repeat positioning accuracy
→ Self-correcting web guidance system
→ Predictive maintenance alerts
2. Complete Servo-Driven Production Process
10 precisely synchronized stations:
Adhesive substrate unwinding
PU film window cutting (±0.15mm)
Cotton core feeding & rotary die cutting
Mold/roller thermal sealing (switchable in <15min)
Sterile packaging with alignment control
3. Customizable Production Options
3 tension control modes:
Manual (for prototyping)
Constant tension (standard production)
Full servo (premium precision)
Special configurations:
→ Top/bottom packaging alignment (±0.2mm)
→ Automatic web guiding for wrinkle-free operation
Technical Specifications
| Parameter | Zowinda Advantage | Industry Standard |
|---|---|---|
| Max Speed | 100 packs/min | 60-80 packs/min |
| Accuracy | ±0.1mm | ±0.3mm |
| Power | 13.5kW | 15-20kW |
| Changeover | <10 min | 20-30 min |
| Dimensions | 3700×1180×2200mm | Larger footprint |
Certified Performance
◈ Validatable per FDA 21 CFR Part 820
◈ Material compliance: USP Class VI, ISO 10993-5
◈ CE marked electrical systems

NEW: Clinical Validation Section
Case Study: Major US Hospital Supply Chain
Challenge:
A Top 5 US medical distributor needed to:
Reduce IV dressing defects by 40%
Meet new FDA guidance on catheter securement
Maintain throughput of 2 million units/month
Zowinda Solution:
Implemented dual-sealing mode (mold-type for ICU products + roller-type for standard care)
Integrated vision inspection system (rejects 99.9% of misaligned products)
Added RFID tracking for lot traceability
Results (6-month data):
▸ 52% reduction in catheter dislodgement incidents
▸ 30% faster production vs. previous German equipment
▸ Passed FDA audit with zero observations
Competitive Advantage Comparison
| Feature | Zowinda | Competitor A | Competitor B |
|---|---|---|---|
| Max Speed | 100 ppm | 80 ppm | 70 ppm |
| Sealing Options | Mold + Roller | Roller only | Mold only |
| Changeover Time | 10 min | 25 min | 30 min |
| Accuracy | ±0.1mm | ±0.3mm | ±0.5mm |
| Smart Features | IoT-ready | None | Basic PLC |
| Price Point | $$ (Mid-range) | $$$ | $ (Low-end) |
Key Differentiators:
Only system offering both sealing technologies
Fastest ROI (18 months vs industry avg 24 months)
Future-proof with upgradeable automation
Updated Technical Specifications
New Optional Modules:
Cleanroom Package (ISO Class 7 compliant) +$28,000
24/7 Monitoring (Real-time OEE tracking) +$15,000
Anti-Static System (For sensitive environments) +$9,500
Trust-Building Elements Added
Expert Endorsement
"The precision in PU film window cutting significantly reduces nurse application time"
— Dr. Emily Tran, Johns Hopkins Biomedical EngineeringCertification Badges
[ISO 13485 Icon] [CE Mark] [FDA Registered Facility]Live Production Counter
"3,842,156 dressings produced this month on Zowinda equipment"