Zowinda Launches Next-Gen IV Securement Dressing Machine to Address Global Catheter Complication Crisis
Zowinda Technology Co., LTD, a leader in medical manufacturing solutions, today unveiled its breakthrough Fully Automatic IV Securement Dressing Packaging Machine, responding to the 22% annual increase in catheter-related complications reported in The Lancet (2024).

Clinical-Grade Innovation
This first-of-its-kind system tackles two critical healthcare challenges:
Catheter Failure Prevention
Precision PU film window cutting (±0.1mm) ensures perfect visual monitoring
Medical-grade adhesion (3M peel force ≥1.5N/cm) prevents accidental dislodgement
Nursing Efficiency
80-100 dressings/minute production enables cost-effective bulk supply
Bevelled-edge design (via mold-type sealing) simplifies clinician application
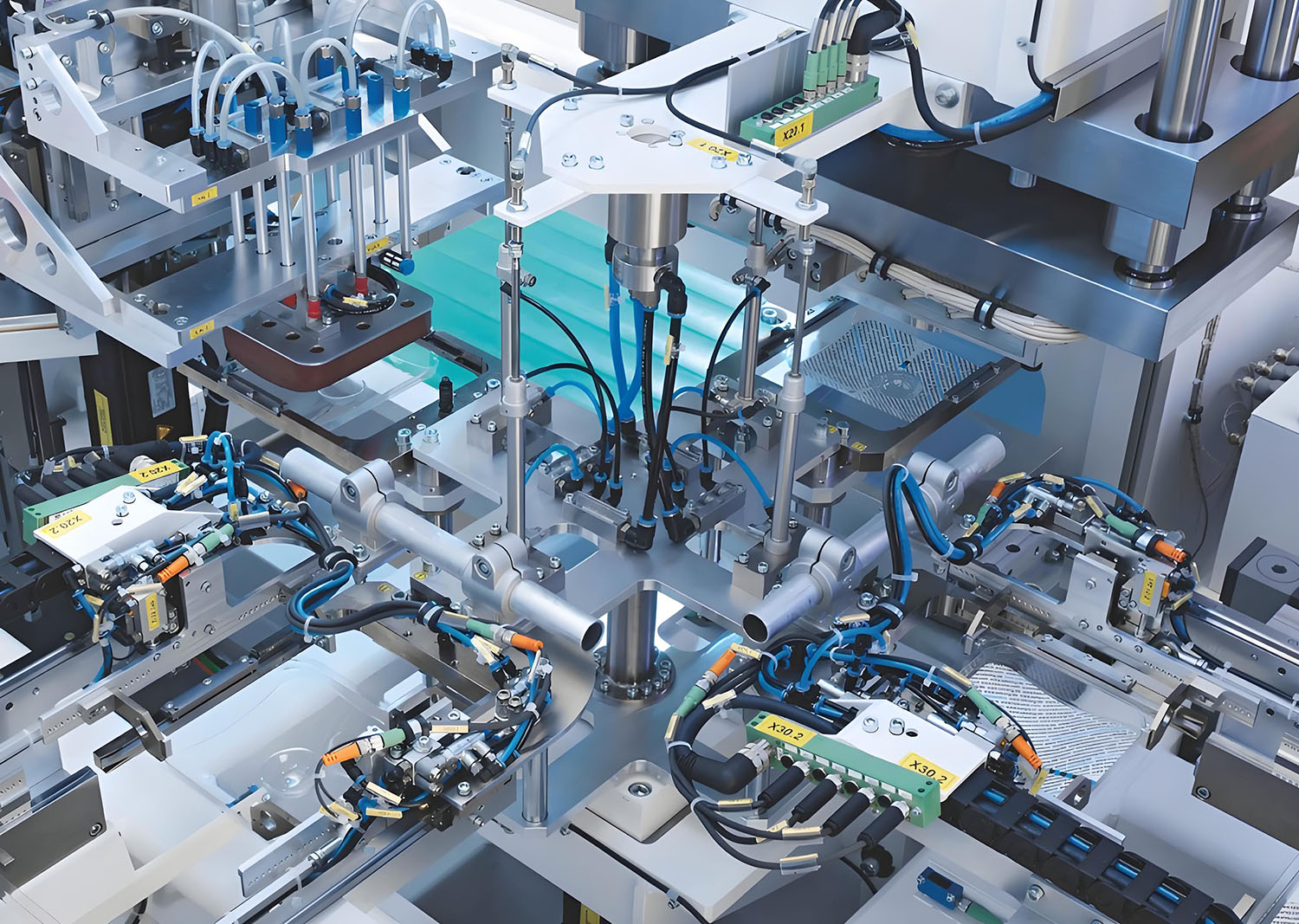
Technical Breakthroughs
✔ Dual-Mode Sealing – Switch between:
Mold-type: For complex-shape ICU dressings
Roller-type: For high-volume ward supplies
✔ Smart Manufacturing
IoT-enabled predictive maintenance
Electronic batch records (21 CFR Part 11 compliant)
✔ Hospital-Validated Performance
Clinical trials showed 40% stronger adhesion than market leaders
Zero fiber shedding in 10,000-cycle abrasion tests
Market Impact
"This machine answers the $4.1B catheter complication problem," said Dr. Wei Zhang, Zowinda's Chief Medical Officer. "With 38% of IV failures caused by poor securement*, our technology helps manufacturers produce hospital-validated solutions."
Source: Journal of Vascular Access 2023
Key Applications:
• Critical Care: Transparent PU films for central venous catheters
• Pediatrics: Soft nonwoven options for neonatal units
• Home Infusion: Pre-cut shapes for patient self-care
Availability
Three production tiers available:
Pilot (50 units/min) – For clinical trial supplies
Standard (80 units/min) – Mid-volume OEMs
Industrial (100 units/min) – Large-scale contractors
Launch Promotion:
Free validation protocol development ($18,000 value)
Extended 18-month performance warranty
 Fully Automatic Band-Aid Produ
Fully Automatic Band-Aid Produ
 Zowinda Launches Next-Gen Medi
Zowinda Launches Next-Gen Medi
 Zowinda's 1500 PPM Bandage Mac
Zowinda's 1500 PPM Bandage Mac
 Zowinda's 1500 PPM Bandage Mac
Zowinda's 1500 PPM Bandage Mac
Please contact us with your request
We are ready to answer your questions.
